What makes us tick? Bio of course...thus the name ProBio'Ticks'. As the writers of this blog , let us introduce ourselves.We are the students of SMK Infant Jesus Convent and we have established a blog called ProBio'Ticks' on 19th February 2015 as we are involved in a school education programme.We are hoping that this blog will benefits our viewers and us through your comments and viewing status.
Wednesday 11 March 2015
Chapter 9 - Endangered Ecosystem ( Thermal Pollution )
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Chapter 9 - Endangered Ecosystem ( Air Pollution )
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Chapter 9 - Endangered Ecosystem ( Nitrogen Cycle )
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Chapter 9 - Endangered Ecosystem ( Green House Effect )
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Chapter 9 - Endangered Ecosystem ( Carbon Cycle )
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Chapter 9 - Endangered Ecosystem ( Noise Pollution )
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Chapter 4 - Chemical Composition Of Cell F4
credit to : PN AIDA ABDUL KARIM our SMKIJC BIOLOGY TEACHER
Friday 6 March 2015
Wednesday 4 March 2015
Form 4- Chapter 3 : Movement of Substances Across the Plasma Membrane
1. Uniqueness of Plasma Membrane /cell membrane
- it is a semi-permeable membrane
- it allows water and certain substances to move in and out of the cell.
2. Importance of Plasma Membrane:
- cells obtain nutrients and gases
- cells excrete metabolic wastes
- cells can maintain pH for enzyme activity
- cells can maintain ionic concentration of the cells for enzyme activity
- control the types and the amount of substances
- allow useful substance (hormones/enzymes) to secrete from cells
- protect cells
- a boundary between the inside and outside of cell.
3. Structure of the basic unit of plasma membrane
- Phospholipid molecule:
‘Head’ – hydrophilic: a polar phosphate molecule (philic~loves water / attracted to water)
‘Tail’ – hydrophobic: two non-polar fatty acids (phobic~hates water / repelled to water)
- Formation:
Hydrophilic heads pointing outwards
Hydrophobic tails pointing inwards
Fluid Mosaic Model (Protein embedded in the bilayer)
Carrier protein
- carrier for some molecules (glucose, amino acids, proteins and nucleic acids)
- controls the movement of ions and particles (Na+, Ca2+ and K+)
- Glycoprotein
Glycolipid
- combination of lipids and polysaccharides
4. Permeability
Permeable (allow to pass through)
- small non-polar molecules (vitamins A, D, E, K, fatty acids, glycerol and steroids)
Impermeable (not allowed to pass through but with help of carrier protein and cellular energy, it is allowed to pass through)
- large polar molecules (glucose, amino acids, nucleic acids and polysaccharides)
- charged ions (H+, Na+, K+, Cl- and Ca2+)
Substances that are allowed to move out of the cell:
- CO2
- excess H2O
- nitrogenous waste
Substances that are allowed to move into the cell:
- O2
- amino acids
- mineral salts
- glucose
Materials must be able to move through the plasma membrane in order for the cell cytoplasm to interact with the external environment. Therefore, the movement of soluble substances can occur in several mechanisms:
-Process of passive transport and active transport
A. Passive Transport
i) Simple Diffusion
- not selective: lipid-soluble molecules, gases and water.
- not control by cell.
- movement of the molecules from a region of higher concentration to a region of lower concentration.
- Factors affecting the rate of diffusion are temperature, size of molecules/ions, diffusion gradient, surface area and diffusion medium.
- example: diffusion of oxygen and carbon dioxide at the alveolus.
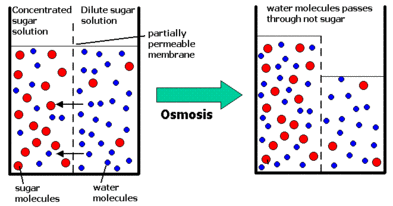
ii) Osmosis
- only water molecules.
- not control by cell.
- movement of water from a region of higher concentration to one of lower concentration and often occurs across a semi permeable membrane.
- strong sucrose solution = less water molecule = low water potential.
- weak sucrose solution = more water molecule = high water potential.
- example: absorption of water by root hairs.
iii) Facilitated Diffusion:
- very specific: glucose, nucleic acids, amino acids, protein and mineral ions.
- control by cell.
- transport of molecules (only certain molecules) across the outer membrane of living cell by a process of carrier protein (hydrophilic group) / channel protein (Ions: Na+, Ca2+, K+) within the cell membrane.
- normally take place from a region with higher concentration of molecules to a region of lower concentration.
example: absorption of digested food in the villus
B. Process of Active Transport
- very specific: minerals ions and amino acids.
- controlled by cell.
- This process needs carrier proteins and energy (due to against concentration gradient) from a region of lower concentration to a region of higher concentration).
- Cell must expend energy that derived from ATP (adenosine triphosphate)
- example: human nerve cells (sodium ions are constantly transport out of the cell) / ions intake by root hairs of a plant.
Type of Solution
- Hypotonic
- Isotonic
- Hypertonic
1) Hypotonic
- Solute concentration in the external solution is lesser than solute concentration inside the cell.
- Water concentration outside the cell is higher than the water concentration inside the cell.
2) Isotonic
- Solute concentration in the external solution is equal to the solute concentration inside the cell.
- Water concentration inside and outside of the cell is the same.
3) Hypertonic
- Solute concentration in the external solution is greater than solute concentration inside the cell.
- Water concentration outside the cell is lower than the water concentration inside the cell.
Type of Solution
|
Hypotonic
|
Isotonic
|
Hypertonic
|
Animal Cell
|
The cell inflates due to the water molecules enter the cell. Eventually it bursts (thin plasma membrane). Example: red blood cell in distilled water.
|
No change in the size of cell. Net movement of water is zero. Example: red blood cell in 0.85% sucrose solution.
|
The cell shrinks and becomes soft and dehydrated due to the water molecule leave the cell. Example: red blood cell in 5% sodium chloride solution.
|
Plant Cell
|
The cell expands and becomes firm / turgid due to the water molecules enter the cell. The rigid cellulose cell wall expands slightly and prevents cell from bursting. Example: strip of potato in distilled water.
|
No change in the size of cell. Net movement of water is zero. Example: strip of potato in 5% sucrose solution.
|
The cell becomes flaccid (plasmolysis occurs), vacuole and cytoplasm shrink due to the water molecules leave the cell. Example: strip of potato in 30% sucrose solution.
|
Application
- Food is soaked in a concentrated salt solution to prevent bacteria and fungus to survive.
- Chemical fertilizer (dissolved ions) increases solute concentration (decrease water molecules) in soil. Therefore, water leaves from the cell sap of the plant which result the plant wither.
Subscribe to:
Posts (Atom)